The virtue of patients
April 10, 2024You can understand the trepidation of first injecting a human patient with a drug in which you have played a significant role in getting to this point.
However, that time came for Argenica and its CEO Dr Liz Dallimore when the first stroke patient was dosed in the company’s clinical trial of ARG-007, after more than a decade of meticulous safety checking and preparation.
The tribulations following a stroke are stark, with only 10 per cent of stroke patients ever making a full recovery. The resulting brain injury following a stroke can lead to lifelong disability.
However, with the first patient dosed at Royal Melbourne Hospital in Argenica’s phase 2 clinical trial, the patient, who had a confirmed diagnosis of a stroke from a large vessel occlusion, may have become the first non-healthy human to be injected with Argenica’s neuroprotective therapeutic.
The start of this trial garners hope that ARG-007 will show success in this trial in reducing brain injury following stroke.
Given the trial is blinded it is currently not known if the patient was dosed with a placebo or ARG-007. The trial will remain blinded until 92 patients are dosed, at which point Argenica will unblind the data to determine whether the drug is both safe, and effective at reducing brain injury.
ARG-007 was first discovered by Argenica Chief Scientific Officer Professor Bruno Meloni, who was probing ways to get different kinds of drugs into the brain.
In his research efforts at the Perron Institute and the University of Western Australia, Meloni found one drug demonstrated some truly gripping potential.
But Western Australia is not The Island of Doctor Moreau, nor Meloni a mad scientist, so a long 10 years of research work was spent building an understanding of the therapy, looking at how the drug works, its safety, and preclinical models for reducing brain injury following an ischemic stroke.
That decade’s efforts were long but fruitful.
ARG-007’s potential was validated, and it was time to form Argenica Therapeutics and get this thing going in earnest, and into public trading and clinical trials.
A company needs a helmsperson, and here enters Dr Liz Dallimore, recruited as the company’s inaugural chief executive.
Dallimore knew Meloni from her time at the Perron Institute, home to the state’s most successful neuroscience research breakthroughs team and was already well versed in stroke research.
She knew the science, but Argenica Non-Executive Director Geoff Pocock says there was much more to the unassuming Dallimore, even with a neuroscience PhD and several years of research by her name.
“More than that, and arguably more importantly, Liz brings an outstanding commercial skill set through extended time in a number of top-tier professional services organisations,” Pocock says.

Dallimore speaking at the Annual Virtual Healthcare Forum
He noted her background, including consulting to a wide range of med-tech and biotech companies through different points of their lifecycles, had provided her with exceptional communication and interpersonal skills.
Skills much needed to establish relationships and find solutions for the complex issues a company like Argenica would have to work through.
“This combination of professional, commercial skills coupled with a strong technical understanding is extremely rare and gives Liz all the tools necessary for success,” Pocock affirms.
But for Dallimore, now Argenica’s CEO and Managing Director, it was less about the title and more about her fascination with work already done.
“There was a huge body of evidence behind the drug and Bruno had really done everything he could to de-risk the asset, so we really just needed to get it into the clinic,” she says.
“Coming into this company with the amount of work that Bruno had done in that preclinical setting was a no-brainer.
“I thought ‘this is absolutely amazing!’ just the level of detail he went into to build that knowledge around the drug asset. But humans are different, and any drug development program does come with risks.”
The first step of drug development is a phase 1 trial, proving the drug is safe and well tolerated in healthy humans.
“That’s what we did in phase one at Linear, a Perth clinical research organisation which runs these early-stage clinical trials. It’s all healthy adult volunteers that come into their facility and they were dosed with our drug,” Dallimore explains.
Safety was confirmed, the data all looked good, and Argenica was able to move into the next phase and test safety in stroke patients.
“That’s where we’re at now and we have just start dosing patients in that phase two trial. The main regulatory hurdle to get a drug approved is safety, that’s first and foremost, but obviously we want to make sure that the drug is having some kind of effect on the ability to protect brain cells following stroke,” Dallimore assures.
“The main thing you need to make sure of is really robust preclinical data to de-risk the drug asset as much as you possibly can before clinical trials, not only from a safety point of view, but efficacy.”
But you never really know until you’re putting a drug into a human being, particularly in an acute setting such as when they’re experiencing a stroke.
“We’ve pretty much done everything we can to de-risk it, but there’s always a little bit of trepidation around moving into clinical trials,” she says.
“A lot of clinical trials fail because of bad trial design. We have done a lot of work to make sure that how we are running the trial is robust and it’s going to give us the best chance of getting the drug approved.
“We would never cut corners to save time, it’s patient safety first and foremost and then just doing everything we can to see that efficacy signal in this stage of the phase two.”
Biotechnology and drug development can be difficult to parse, often filled with the acronyms and jargon which can seclude lazier minds than Dallimore’s.
But if you think that every investor involved in the space is well informed and across the science, you would be wrong.
“No, they’re generally not informed, it’s difficult science!” Dallimore chuckles.
Thankfully, she finds her work easier to explain than some.
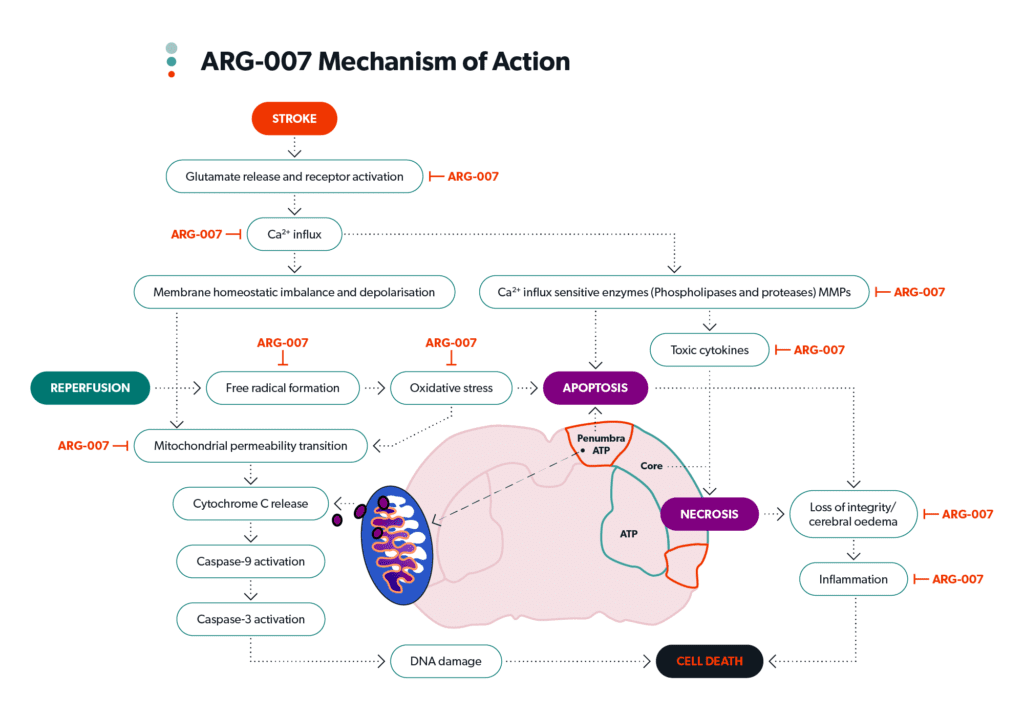
“People know what a stroke is, and I can paint a visual that it’s a clot in the brain that stops blood being delivered,” Dallimore illustrates.
“Brain cells can only last about three seconds without delivery of the oxygen and the nutrients they need, and brain cells start dying almost immediately.
“Our drug essentially hibernates those brain cells and stops them from dying, so it’s a pretty easy narrative in terms and people get that.”
Dallimore assures us there are certainly people who want to delve into the science, and she is fortunate to have so much data for investors to sink their teeth into.
And there is a wealth of data on more than stroke, with ARG-007 having shown preclinical effectiveness in treating a range of other neurological impairments.
Beyond stroke, the priorities are traumatic brain injury, Alzheimer’s disease, and hypoxic ischemic encephalopathy.
For the latter condition – a rare paediatric condition caught by infants around childbirth – ARG-007 has been granted an Orphan Drug Designation and Rare Pediatric Disease Designation by the United States Food and Drug Administration.
These designations are a way to incentivise companies to invest in, research and develop treatments for rare conditions.
And for Argenica, it provides critical ongoing interaction with the FDA, helping design clinical trials, and seven years of market exclusivity in the US.
For stroke, Argenica hopes to submit an Investigational New Drug Application within the year, and with that process seek to fast track a phase 3 clinical trial in stroke.
“An IND and fast-track designation means we get more touchpoints with the FDA, and they’ll prioritise looking at our clinical trial data, so if we’re successful in getting that fast-track designation for stroke, that’s another really good thing that we can leverage with the FDA to accelerate the approval of the drug in the stroke indication,” Dallimore notes.
But the focus now is lasered in on trials. Three hospitals have been fully activated, with another seven hospitals coming online over the next couple of months.
“It is quite a unique trial, being run in an acute emergency setting with patients getting rushed in,” Dallimore says.
“We’re really relying on our principal investigator neurologists at each of these hospitals to recruit those patients in. Our job is to keep people engaged and motivated around the trial.”
The Argenica leader expects it will take a couple of years before final data readout on the trials, so there is a bit more waiting yet to be done.
But after more than a decade’s hard work, she already understands well the virtue of patience.
Please note the following valuable information before using this website.
Independent Research
Market Open Australia is intended to be used only for educational and informative purposes, and any information on this website should not be taken as investment advice or guidance. It is important to conduct your own research before making any investment decisions, which should be based on your own investment needs and personal circumstances. Any investment decisions based on information contained on this website should be taken in line with independent financial advice from a qualified professional or should be independently researched and verified.